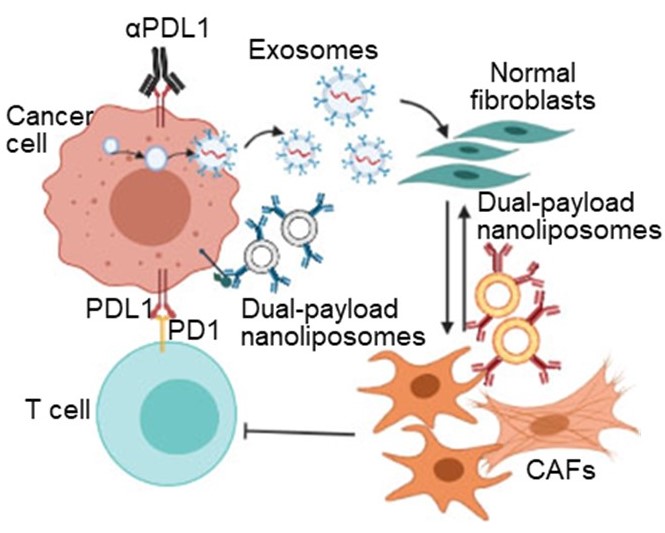
Modulation of Tumoral Exosomal Machinery to Suppress Differentiation of Stromal Fibroblasts
Cancer cells program fibroblasts into cancer associated fibroblasts (CAFs) in a two-step manner. First, cancer cells secrete exosomes to program quiescent fibroblasts into activated CAFs. Second, cancer cells maintain the CAF phenotype via activation of signal transduction pathways. We rationalized that inhibiting this two-step process can normalize CAFs into quiescent fibroblasts and augment the efficacy of immunotherapy.
Learn more…
We showed that cancer cell–targeted nanoliposomes that inhibit sequential steps of exosome biogenesis and release from lung cancer cells block the differentiation of lung fibroblasts into CAFs. In parallel, we demonstrated that CAF-targeted nanoliposomes that block two distinct nodes in fibroblast growth factor receptor (FGFR)–Wnt/β-catenin signaling pathway can reverse activate CAFs into quiescent fibroblasts. Co-administration of both nanoliposomes significantly improved the infiltration of cytotoxic T cells and enhances the antitumor efficacy of αPD-L1 in immunocompetent lung cancer–bearing mice.
Genetic Engineering of CAFs into In Situ Factories of Cytolytic & Immunomodulatory Exosomes
The dense stroma, dominated by CAFs and their secreted extracellular matrix (ECM), creates a biophysical and functional barrier that impedes immune cell infiltration and supports tumor growth. Our innovative approach seeks to transform this challenge into a therapeutic opportunity by genetically engineering CAFs to produce cytotoxic extracellular vesicles (EVs) that specifically target and kill PDAC cells.
Learn more…
We hypothesize that CAF-derived EVs can be harnessed to deliver cytotoxic proteins specifically to PDAC cells, bypassing the need for immune cell infiltration. Our strategy leverages the unique localization of CAFs within the TME wrapping the tumor vasculature and their inherent gene expression stability to produce EVs loaded with cytotoxic proteins, fused to EV constitutive marker and targeted to PDAC cells via tumor specific scFv. This design ensures selective delivery and activation of the cytotoxic protein within cancer cells.

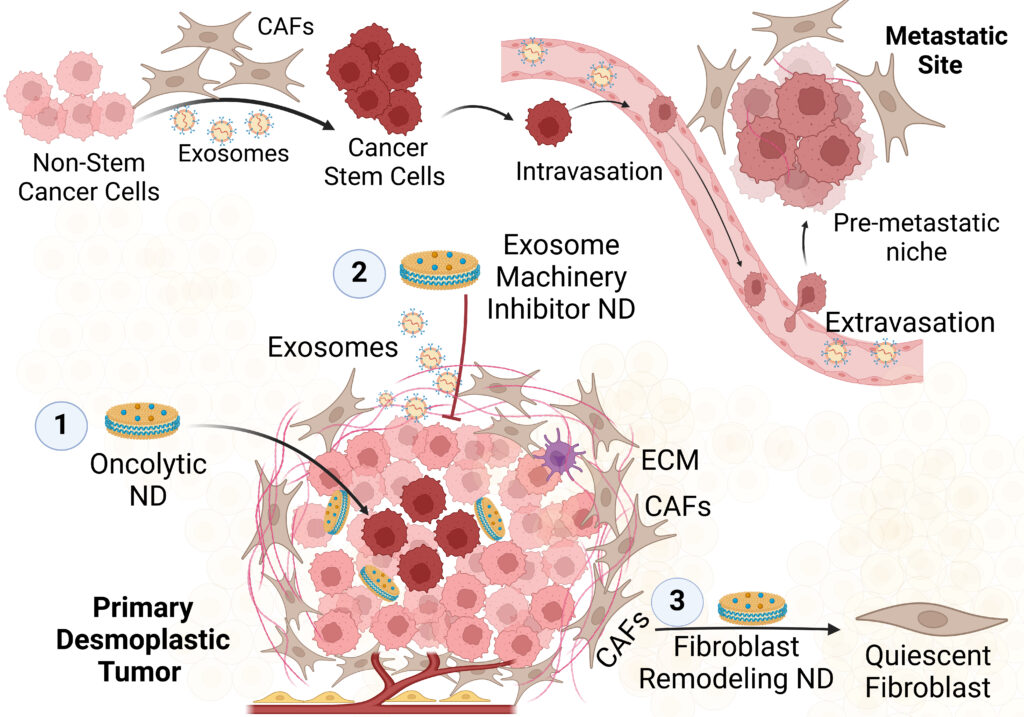
Remodeling of CAFs Enhances Oncolytic Immunotherapy to Overcome Stemness in Desmoplastic Tumors
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and deadliest forms of cancer. While major progress in immunotherapy has been made in recent years, it remains poorly effective in this malignancy. The immune resistance of PDAC stems from low immunogenicity of PDAC cells as well as the inaccessible cells that reside deep in the tumor core. Second, pancreatic cancer associated fibroblasts (CAFs) fuel stemness and metastasis of PDAC cells by suppressing their immune recognition and establishing pre-metastatic niche at distant organs to help their colonization.
Learn more…
Therefore, we engineered ultrasmall circularized nanodiscs (cND) that encapsulate oncolytic peptide LTX-315 and fibroblast remodeling Notch signaling inhibitor. The engineered oncolytic LTX-315 loaded cND successfully induced immunogenic cell death (ICD) of immune resistant pancreatic cancer cells. The ultrasmall size (9 nm) of cND enabled its deep tumor penetration to eradicate resistant cancer cells. In parallel, the Notch blocking cND promoted quiescence of the pancreatic CAFs as denoted by downregulation of SMA, FAP and collagen. In KPC and orthotopic syngeneic PDAC mouse models, the dual oncolytic/Notch blocking cND combined with aPD-1 therapy significantly suppressed tumor growth and prevented its metastasis compared to vehicle-treated mice.
Targeting the Immunoregulatory Role of Fibroblasts in Autoimmune Disorders
Many underlying factors contribute to the variable response of rheumatoid arthritis (RA) patients to current therapies. Synovial fibroblasts (SFs) and macrophages (SMs) have a major pathological role in RA progression and markedly contribute to drug resistance, but current RA drugs do not target SFs.
Learn more…
We hypothesize that blocking the upregulated signaling pathways in SFs will switch their activated phenotype into a quiescent one that dampen inflammation and reverses joint fibrosis. Furthermore, inducing repolarization of the inflammatory M1-type macrophages to anti-inflammatory M2-type will relieve joint inflammation. We propose to engineer ultrasmall nanodiscs (NDs) to simultaneously induce quiescence of the activated SFs and repolarize M1 pro-inflammatory SMs to M2 anti-inflammatory phenotype

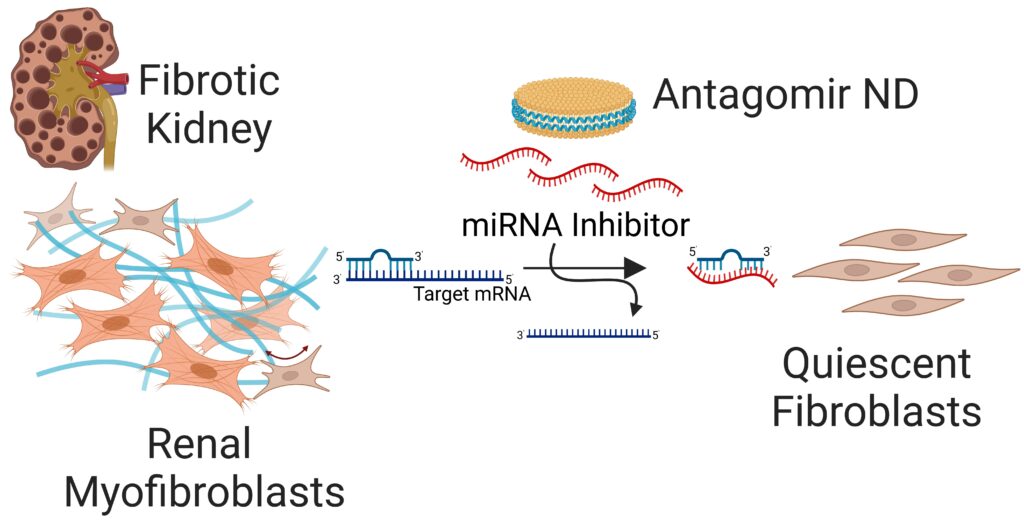
Targeted Gene Delivery Approaches for Phenotype Reprogramming of Myofibroblasts in Fibrotic Diseases
We are engineering novel types of nanomedicines for targeted delivery of antagomirs and siRNA to interfere with the upregulated profibrotic signaling pathways in the matrix-producing myofibroblasts in kidney and pulmonary fibrosis. Targeted inhibition of those pathways reprograms the differentiated phenotype of myofibroblasts into a quiescent one thereby halting matrix production and fibrosis progression.